

Production
Here at Gentech, we are working to produce the most efficacious and safe therapies in the history of medicine. And, as ever, our goal of putting patients first is what drives these innovations.
Production Process

The first step in the recovery of an antibody from a mammalian cell culture is harvest (removal of cells and cell debris to yield a filtered fluid suitable for chromatography), which is accomplished through use of centrifugation.
Protein A-based chromatography is used to obtain a high degree of purity and recovery in a single step. The result yields a relatively pure product that only requires removal of a small proportion of process and product related impurities. In addition, to achieve viral clearance, a low pH hold is included in the process.
Two additional chromatography steps are employed as polishing steps, incorporating cation and anion exchange chromatography. These steps provide additional viral, host cell protein and DNA clearance, removal of aggregates, and other minor contaminants.
Lastly, the purified product is concentrated and dissolved into the final formulation.
2,3
2,3
2,3
2,3
Regulatory Approvals

USA
In 1998, Herceptini® trastuzumab was granted Food and Drug Administration (FDA) approval to introduce or deliver for introduction into interstate commerce Trastuzumab under Department of Health and Human Services U.S. License No. 1048
Singapore
In 2003, Singapore’s Health Sciences Authority (HSA) approved the use of Herceptini® trastuzumab for the treatment of HER2-positive breast cancer.
Japan
In 2008, Japan’s health authorities approved Gentech’s cancer drug Herceptini® for the treatment of HER2-positive breast cancer.
Australia
In 2010, based on a review of quality, safety and efficacy, Therapeutic Goods Administration (TGA) approved the registration of Herceptini® trastuzumab.
Clinical Trials Results
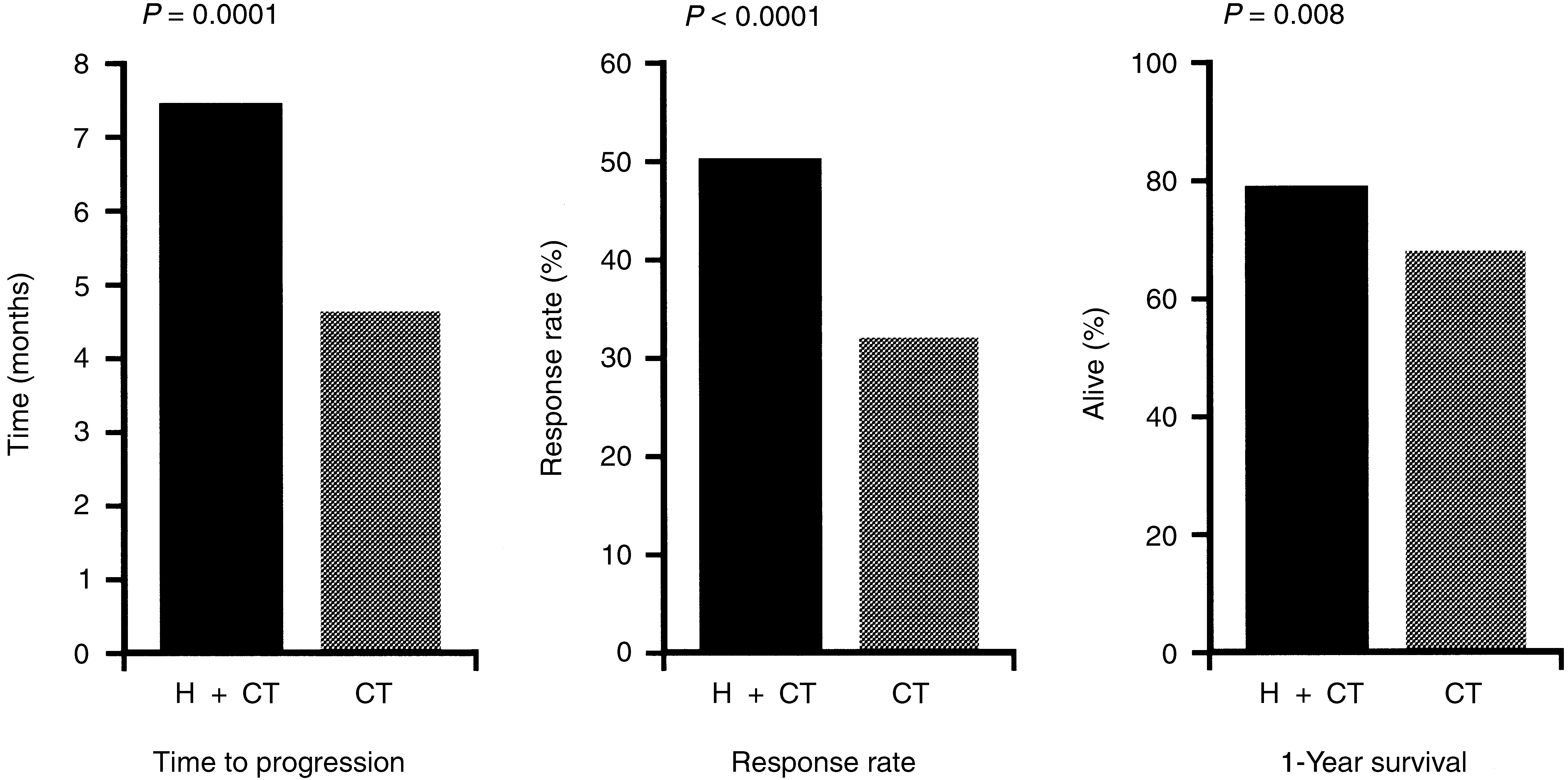
(Eiermann, 2001)
Between June 1995 and March 1997, an international, randomized, Phase III, open-label, clinical trial was conducted in 469 patients with metastatic breast cancer evaluating the safety and efficacy of Herceptini® trastuzumab monotherapy in treatment of women with metastatic breast cancer who had not previously been treated with chemotherapy for their metastatic disease.
The median time to disease progression in the group assigned to chemotherapy plus trastuzumab was 7.4 months, whereas in the group given chemotherapy alone it was 4.6 months.
In comparison with chemotherapy alone, treatment with chemotherapy plus trastuzumab was associated with a significantly higher rate of overall response (50% vs. 32%) and one-year survival (78% vs. 67%).
1
1
1
References
1. Eiermann, W. (2001a). Trastuzumab combined with chemotherapy for the treatment of HER2-positive metastatic breast cancer: Pivotal Trial Data. Annals of Oncology, 12. doi:10.1093/annonc/12.suppl_1.s57
2. Liu, H. F., Ma, J., Winter, C., & Bayer, R. (2010). Recovery and purification process development for monoclonal antibody production. mAbs, 2(5), 480–499. https://doi.org/10.4161/mabs.2.5.12645
3. National Research Council (US) Committee on Methods of Producing Monoclonal Antibodies. Monoclonal Antibody Production. Washington (DC): National Academies Press (US); 1999. 5, Large-Scale Production of Monoclonal Antibodies. Available from: https://www.ncbi.nlm.nih.gov/books/NBK100189/